Periodic Trend in Metal Reactivity Down a Group
Use the solubility pattern observed for the known and unknown alkaline earth compounds in Part B to deduce the identity of the unknown alkaline earth metal. Period - reactivity decreases as you go from left to right.

Group 1 Alkali Metals Alkali Metal Physical Properties Physics
Energy levels increase valence electrons are farther away from protons decreases attraction and.

. Ity of these metals increases going down the group ie sodium is more reactive towards water than lithium. As we go down the group the atom gets bigger. Since the ionization energy decreases going down a group or increases going up a group the increased ability for metals lower in a group to lose electrons makes them more reactive.
The periodic trend in the solubility of alkaline earth metal compounds is that as one goes down a group it increases in activity. The metallic character increases as you go down a group. The valence electrons are less tightly.
Periodic Trends in Reactivity Introduction The structure of the periodic table is such that elements with similar properties are aligned vertically in columns called groups This leads to trends in properties such as electron affinity and atomic radius as one moves both down the periodic table within a specific group or as one moves horizon-. Why does reactivity increase as you go down the periodic table. In metals down the group reactivity increases whereas in non metals down the group reactivity decreases.
Nov 17258 PM Reactivity Trends Metal reactivity increases down a group because as nuclear shielding increases and the nucleus hold on the valence electron weakens therefore it is easier to remove valence electrons. As you go down a group the atomic number increases. As we go down the group the atom gets bigger.
The outer electrons are easier to remove as they are further from the nucleus and there is more shielding resulting in a lower nuclear attraction. Reactivity Trend in the Periodic Table The most reactive element is fluorine the first element in the halogen group. Describe and explain the trend in reactivity down the alkali metals group.
Corner of the periodic table. Therefore the attraction between the nucleus and the last electron gets weaker. The bigger the atom.
From this we know that Option C Ba is located the most left and farthest. Reactivity increases down the group. How can the observed periodic trends in reactivity of the elements be explained.
Group - reactivity increases as you go down a group In Non-metals Period - reactivity increases as you go from the left to the right. Keeping this in consideration what is the trend of reactivity of metals across a period. The overall trend for the reactivity of Group 2 metals with water is an increase down the group.
How do you determine chemical reactivity. This makes it easier for the atom to give up the electron which increases its reactivity. Nonmetal reactivity decreases down a group because the nucleus ability.
Within each group of metals reactivity increases as you go down the group. What happens when you go down a column on the periodic table. Why is the periodic trend for reactivity opposite for metals and non metals.
Period - reactivity increases as you go from the left to the right. Potassium is more reactive towards water than sodium etc. The least reactive elements are the noble gases.
The most reactive metal is francium the last alkali metal and most expensive element. Keeping this in view what is the trend in reactivity. This rank-ing in reactivity Li Na K is in accord-ance with periodic law and can be predicted by periodic trends in first ionization energies IE 1.
In metals the periodic trend in reactivity increases as you move to the left of the periodic table and down a group. Keeping this in view how does reactivity change as you go down a metal group. Trends in metal reactivity DOWN A GROUP increases down a group.
Period - reactivity decreases as you go from left to right. Reactivity Trends Metal reactivity increases down a group because as nuclear shielding increases and the nucleus hold on the valence electron weakens therefore it is easier to remove valence electrons. This is because the outer electron in each atom is further away from the nucleus as you go down the group and so the electrostatic forces between the nucleus and the outer electron are weaker.
For example barium has more activity than strontium or calcium.

Interactives The Periodic Table Groups Chemistry Classroom Science Chemistry Chemistry Notes

Gcse Ccea Chemistry The Periodic Table Complete Revision Summary Teaching Resources Gcse Science Revision Chemistry Gcse Chemistry Revision

Familia 7 Chemistry Lessons Science Chemistry Teaching Chemistry

Periodic Table Trends Chemistry Homework Page Unit Bundle Chemistry Worksheets Teaching Chemistry High School Chemistry

Nitrogen Facts Properties Uses Discovery In 2021 Chemistry Lessons Study Chemistry Chemistry Basics
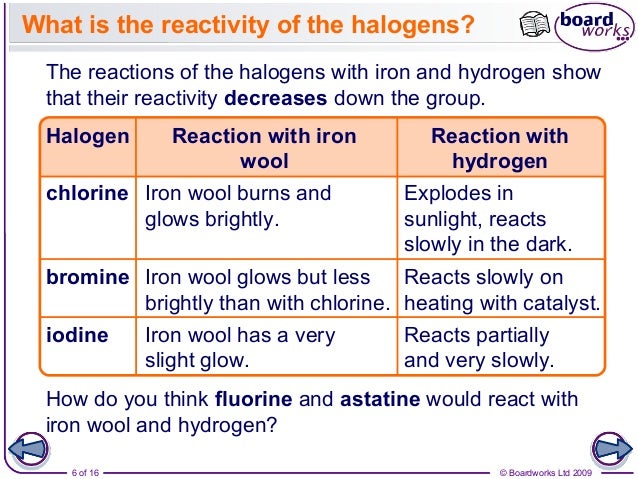
Comparing Halogen Reactivity Trends With Those Of The Alkali Metals Halogens Become Less Reactive Down The Chemistry Classroom High School Chemistry Chemistry

Chemistry Science Reactivity Series Lesson Activities Chemistry Lessons Study Chemistry Chemistry Basics

Give Your Students A Visual Introduction To The Families Of The Periodic Table This Produc Informational Text Informational Texts Activities Chemistry Lessons

The Periodic Table Of Oxidation States Oxidation State Teaching Chemistry Chemistry Lessons

Alkaline And Alkaline Earth Metals Alkaline Earth Metals Alkaline Chemistry Education

Compound Interest Teaching Chemistry Chemistry Classroom Chemistry Education

Pin On Gcse Chemistry Revision Notes

Comparing Halogen Reactivity Trends With Those Of The Alkali Metals Halogens Become Less Reactive Down The Chemistry Classroom High School Chemistry Chemistry

Alkali Metals Properties Periodic Table Ios App Chemistry Periodic Table App

A Section Of The Periodic Table Moving Across Period Two Elements Have The Electronic St Periodic Table Periodic Table Picture Periodic Table Of The Elements

Alkali Metals Alkali Metal Teaching Chemistry Metal Chemistry

Periodic Table Blocks Of Elements Periodic Table Blocks Periodic Table Chemistry Periodic Table

Types Of The First Order Reaction Chemical Kinetics Reactions Chemistry
Comments
Post a Comment